Astin
10 mg Tablets
20 mg Tablets
Composition :
Astin 10 mg : Each tablet contains 10 mg Ebastine
Astin 20 mg : Each tablet contains 20 mg Ebastine
Pharmacological and Pharmacodynamic Properties:
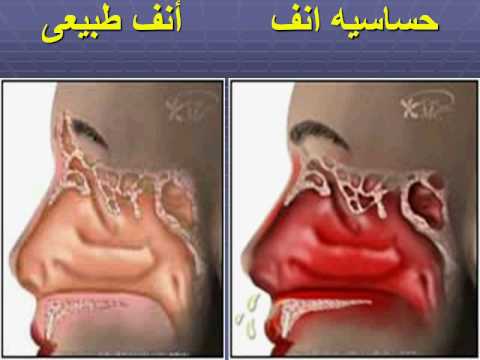
Astin and its active metabolite carebastine are selective histamine HI peripheral receptor antagonists without CNS action & anticholinergic effects
Pharmacological Properties:
Absorption:
Ebastine is rapidly absorbed & almost totally converted into the pharmacological active metabolite carebastine .Concomitant administration of Ebastine with a fatty meal increases the absorption of carebastine of about 50 %.After a single 10 mg oral dose peak plasma level occurs at 2 to 4 hours & achieves levels of 80 to 100 ng/m!. The half-life of carebastine is between 15 and 19 hours with 66% of the drug being excreted in the urine mainly as conjugated metabolites. Following the repeated adminstration of 10 mg once Iday in a single dose. steady state is achieved in 3 to 5 days with peak plasma level 100 to 130 ng/m!.
– Ebastine and Carebastine are highly protein bound >95%
– Ebastine and Carebastine weakly cross the blood brain barrier –
– Excretion in human milk has not been studied
– In elderly patients,no statistically significant changes were observed in the
pharmacokinetics compared to those {If young adult volunteers
– In patients with renal insufficiency ,the elimination half-life of carebastine is
increased to 23 to 26 hours
– In patients with hepatic insufficiency the half-life of carebastine is increased
to 27 hours
Therapeutic Indications:
– Allergic rhinitis
(Seasonal & perennial) whether or not associated allergic conjunctivitis
– Chronic idiopathic urticaria
Dosage and Method of administration:
– Allergic rhinitis: 1 tablet 10 or 20 mg / day according to symptoms severity
– Urticaria: I tablet 10 or 20 mg I day
The safety and Efficacy of Astin in children less than 12 years has not been
established
Contraindications:
– Patients with a known hypersensitivity to Ebastine or any of its ingredients.
– Not to be used during pregnancy and lactation
– Not to be used with patients below 12 years in age
Warnings:
As with most other antihistaminics ,it must be taken with caution when using
Astin in patients kown to have the following conditions:
– Long QT syndrome, hypokalemia,treatment with any drug known to produce
an increase in QT interval or inhibit CYP 3A4 enzyme systems such as azole
antifungals and macrolides antibiotics
Interactions (with other drugs & other forms of interactions) :
– Combinations are not recommended with ketoconazole & erythromycin, both
known to prolong the QT interval
Pregnancy & Lactation :
Pregnancy:
The safety of Astin during pregnancy has not been established, therefore, Astin
is not recommended during pregnancy.
Lactation:
Astin is not recommended for nursing women because it is not known whether
Astin is excreted in human milk or not
Side Effects:
Headaches, dry mouth and drowsiness Rarely abdominal pain, dyspepsia, asthenia, pharyngitis, epistaxis, sinusitis, nausea and insomnia have been reported. Allergic manifestations may occur.
Overdosage:
There is no specific antidote for Ebastine, gastric lavage, monitoring of vital
functions including ECG and symptomatic treatment should be carried out.
Package:
Astin 10 mg: Box contains a strip of 10 tablets and insert
Astin 20 mg: Box contains a strip of 10 tablets and insert
Storage:
– Store at room temperature 25° C or below
– Keep out of reach of children
produced by :
Alkan Pharma S.A.E. 6th of October City – Egypt.

