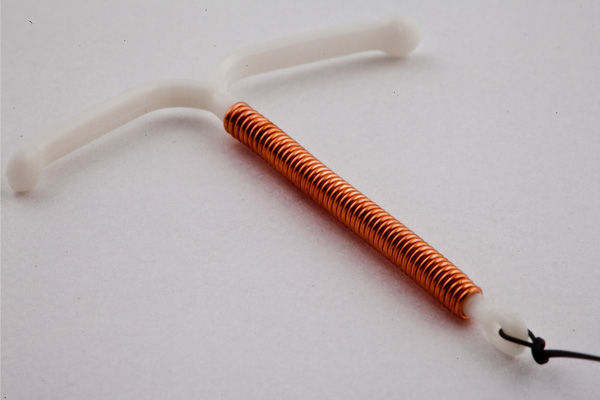
Silver line intrauterine contraceptive device Cu 380 Ag/200 Doctor instruction slip
PROCEDURES FOR INSERTION CAUTION:
– Do not pick up and use any component that has fallen on the floor or table.
– Do not pour the contents of the pouch in the instrument tray.
– Do not use the solid rod to measure uterine cavity length.
A) PREPARING THE USER:
– Operator should wear sterile gloves and use aseptic technique. He/She should gently explain to the client what he/ she is doing.
– Prior to insertion the vagina and cervix should be cleansed with an antiseptic solution.
– The cervix should be visualizes by means of speculum and its anterior lip grasped with a tenaculum. Gently traction on the teanculum will tend to reduce the angle between the cervical canal and endometrial cavity and will greatly facilitate introduction of the uterine sound. The tenaculum should remain on the cervix, throughout the insertion of .the Silverline so that gentle traction on the cervix can be maintained.
– The uterine sound should then be introduced in the endocervical cavity until it reaches the fundus. As soon as the direction and length of the cervical canal and endometrial cavity have been
determined, the Silverline may be prepared for insertion.
B) LOADING· SILVERLlNE Cu 380 Ag/ 200 Ag :
Do not open the sterile package or pull the arm of “Frame” in to the insertion tube until immediately before it is introduced into the uterus. The Silverline Cu 380 Ag/200 Ag can be prepared for insertion inside the sterile package as perthe instructions given below.
STEP 1
Ensure Vertical arm of frame is fully inside the insertion tube and the opposite end of the insertion tube should be closer to the package bottom seal.
STEP 2
Place the package on a clean, hard, flat surface, partially open the plastic covering from the end marked ‘OPEN’ till half way to the yellow flange. However, IUD and insertion tube are not to be withdrawn, While holding the tube firmly with one hand, release the threads from flange and draw the device into the insertion tube by grasping both the threads and gently pulling the device into the insertion tube until the knobs at the ends of horizontal arm cover the opening of the tube.
STEP 3
Steadying the flange with one hand, pull the insertion tube until the lower edge of the flange indicates_the measure obtained with the uterine sound, on the scale printed on insertion tube
STEP 4
Holding the package with open end up, and the flaps away from each other, holding the threads slightly stretched with one hand, . 3 put the solid rod into the insertion tube to almost touch the bottom of pulled frame. This will ensure that the threads are lying straight in the tube and will not be disarranged by the solid rod. Be careful not to touch the tip of solid rod or brush against another surface as
this could lead to the solid rod losing it sterility. Ensure that the flange indicates the direction in which the horizontal arm will open in the uterus.
STEP 5
The Silverline Cu 380 Ag/ 200 Ag is now ready for insertion. Peel the remaining cover of the package and lift the loaded tube, keeping it horizontal so that the frame or solid rod does not fall out and also be careful not to dislodge the frame by pushing the solid rod upward. Do not let insertion assembly touch any unsterile surface that may contaminate it.
C )INSERTING THE LOADED SILVERLlNE Cu 380 Ag/ 200 Ag
STEP 1
Gently introduce the loaded insertion assembly through the cervical canal and advance upward until flange comes into contract with cervical os. Ensure that the flange is in the horizontal plane
STEP 2
Holding the solid rod stationary by one hand withdraw the insertion tube by your free hand to touch ribbed part of solid rod thereby the flange is removed from cervical os as well (approx 1.5 cm). The arms of Frame are now unfolded
STEP 3
Advance the insertion tube until the flanqe is touching the cervical os again. The Silverline Cu 380 Ag/200 Ag is now in contact with fundus
STEP 4
To release the device entirely from the insertion tube, hold the solid.rod firmly and draw the tube back’ as far as the backstop
STEP 5
First, gently withdraw the solid rod (hold the insertion tube stationery while removing the solid rod) and then the insertion tube from the cervical canal to prevent pulling the device from the fundal position. Cut the threads so that they are visible only 3-4 cm outside the cervix as shown in fig.
STEP 6
Assist woman from the table slowly (be alert to possible dizziness) and instruct her how and when to check threads. Have her check the threads. Invite questions and instruct about return visit as well as
what to do whom and how to contact for the help if needed.
D) REMOVAL INSTRUCTIONS :
Silvertine Cu 380 Ag/ 200 Ag must be removed by a trained health care provider. This can be done easily and safely in the clinic and takes only a few minutes. Removal is effected by pulling gently at one of the exposed threads. Excessive force in pulling the threads could result in breakage of threads. Some cramping or bleeding may be experienced during removal.
E) The sterile Silverline Cu 380 Ag/200 Ag is for single use only and should not be reused.
F) On completion of shelf life or on removal after use, dispose the items as per the local regulations governing disposal on non recyclable waste/medical waste.
DIRECTIONS FOR IUD USERS :
– Longer and heavier menstrual periods, or bleeding or spotting between periods may occur during the first week after insertion. If they continue or are severe, report to the clinic.
– Cramping may occur following insertion, usually for short time, but could last for several hours to even days. This can be relieved by taking mild analgesic tablets, using hot compresses on abdomen, and/or exercising moderately.
– Check periodically, and particularly after menstruation, to make certain that the threads still protrude from the cervix. If threads are missing, shorter or longer, return to the clinic.
– If Silverline Cu 380 Ag/ 200 Ag is expelled, return to the clinic. There is no continuing protection after expulsion.
– Return to the clinic for check up or for the replacement of the Silverline Cu 380 Ag/ 200 Ag (end of five years and end of three years after insertion respectively), as instructed by physician.
– If your periods is delayed (with symptoms of pregnancy, such as nausea, tender breasts, etc … ) report immediately to the clinic. .
– If there is abdominal pain, pain during intercourse, infections (such as gonorrhea), abnormal discharge, fever, chills consult your physician.
MECHANISM OF ACTION :
Silverline IUD’s act by greatly reducing the likelihood of fertilization. Data and analysis indicate that the main antifertility effect of copper bearing IUD’s involve inhibition of egg or sperm transport and/or the capacity of sperm to fertilize egg. Reduced gamate transport and capacitation inhibits fertilization and occurs before the ovum reaches the uterine cavity. Continuous copper release in uterine cavity from the copper wire with silver core enhances the contraceptive effect of Silverline Cu 380 Ag/200Ag.
FOLLOW UP GUIDELINE FOR PHYSICIANS :
The physician should encourage the user to come for a follow up visit in case of any problem or doubt regarding usage of Silverline Cu 380 Ag/280 Ag. During follow up the physician should pay particular attention to the following points.-
– Heavier bleeding, indicate the possibility of anemia.
– If pregnancy has occurred and the threads are still visible, the Silverline Cu 380 Ag/ 200 Ag should be removed.
– There is a decrease in the risk of ectopic pregnancy among users of Silverline Cu 380 Ag/ 200 Ag due to an overall reduction in the chance of pregnancy. However, should pregnancy occur, it is more likely to be ectopic than in non users.
– Removal of Silverline Cu 380 Ag/ 200 Ag is advisable, if user is exposed to conditions that substantially increase the risk of pelvic inflammatory disease.
CONTRAINDICATION :
– Pregnancy.
– Acute Pelvic Inflammatory Disease.
– Active STD or other lower genital-tract infection.
– Known or suspected malignancy of the genital tract, including undiagnosed vaginal bleeding.
– Post partum endometriosis.
– An infected abortion in the last 3 months.
MANUFACTURED BY :
Pregna international LTD INDIA

